Course Summary
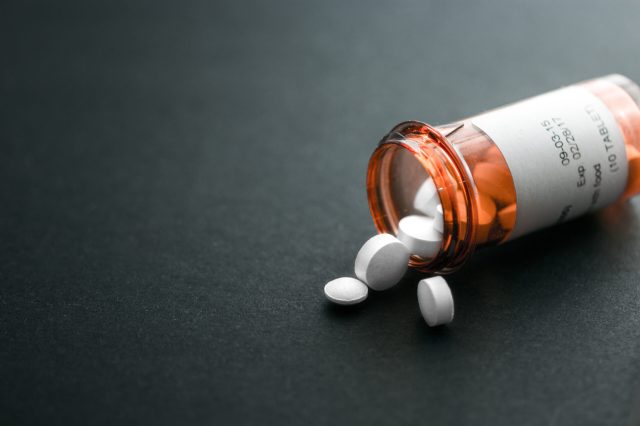
Droperidol is a sedative-hypnotic that has been approved for the treatment of postoperative nausea and vomiting (PONV). The drug is structurally similar to haloperidol, but its use has been limited due to its increased risk of cardiac adverse events involving QT prolongation and torsades de pointes. Off-label use of droperidol typically involves the treatment of patients with acute, undifferentiated agitation in psychiatric and emergency settings, migraine pain, and as an adjunct to general anesthesia. Droperidol, alone or in combination with other drugs, has been used successfully for decades to treat nausea and vomiting. However, clinicians are cautioned to be aware of droperidol complications and their role in causing neuroleptic malignant syndrome. Much of the case studies on droperidol toxicity are found in the emergency medicine literature; however, it is important to investigate potential confounding factors affecting patients who may have been using other drugs or have an undiagnosed pre-existing cardiac condition. While there is the potential for a rare toxic response to droperidol, the possibility of drug toxicity occurring from other causes cannot be excluded completely as a contributing factor. Similar to any antipsychotic drug, tapering of droperidol should be gradual to avoid adverse effects that can occur from abrupt discontinuation.
Course Format
Homestudy
Course Syllabus
- Introduction
- Pharmacological Profile and Uses
- Available Forms and Dosing
- Common Uses of Droperidol
- Nausea and Vomiting
- Acute, Undifferentiated Agitation
- Dosing Adjustment: Geriatric Patients, Hepatic Impairment
- Drug Warning and Contraindications
- Cardiovascular
- Anticholinergic Effects
- Central Nervous System
- Parkinsonism
- Tardive Dyskinesia
- Gastrointestinal and Hepatic
- Hematologic
- Neuroleptic Malignant Syndrome
- Temperature Regulation
- Ophthalmic
- Pheochromocytoma
- Prolactin Levels
- Seizure Disorder
- Venous Thromboembolism
- Falls
- Withdrawal/Discontinuation
- Pregnancy and Breastfeeding
- Drug-Drug Interactions and Toxicity
- Case Study: Droperidol
- Discussion
- Summary
Author
Harpreet Ghai, MS (Pharm), BPharm, Rph
Harpreet Ghai has been a practicing Pharmacist for 12 years in Comox, British Columbia. He has also completed his Master’s in Science, specializing in Medicinal Chemistry from NIPER (National Institute of Pharmaceutical Education and Research), in India. He has spent 3 years in Pharmaceutical Research at Ranbaxy in Gurugram (India), where he was engaged in Structure-based drug designing and was a part of a premier research-intensive group responsible for discovering novel drugs. Harpreet enjoys the day-to-day challenges at the retail pharmacy. Interactions with customers, listening to their drug-related issues, and providing solutions are profoundly rewarding.