Course Summary
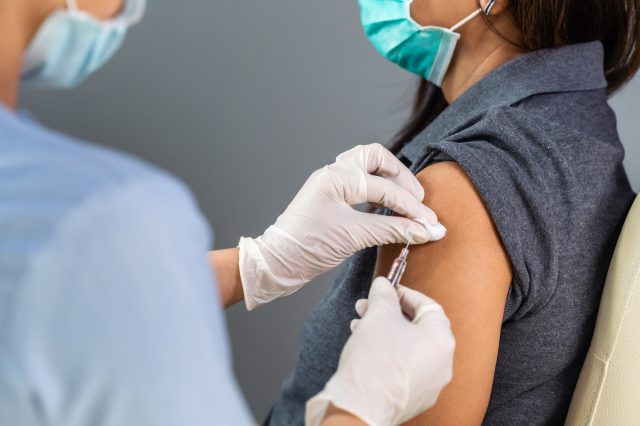
A new coronavirus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the appearance of coronavirus disease 2019 (COVID-19), which has become a global pandemic. This positive-sense single-stranded RNA virus is related to the Severe Acute Respiratory Syndrome (SARS) and the Middle Eastern Respiratory Syndrome (MERS) that appeared in 2002 and 2012, respectively. Medical scientists around the world have focused their research efforts on developing treatments and vaccines to slow the spread and impact of COVID-19. Out of these efforts, three vaccines developed by Pfizer-BioNTech, Moderna, and Janssen (Johnson & Johnson) were authorized by the United States Food and Drug Administration for emergency use for the prevention of COVID-19. These vaccines have different handling and administration requirements that health clinicians must know and understand to promote their efficient and proper use. The Centers for Disease Control and Prevention (CDC) has published guidelines for COVID-19 vaccine storage and handling, as well as public information for health clinicians and recipients of the vaccine relative to safety and the reporting of any adverse side effects from these vaccines.
Course Format
Homestudy
Course Syllabus
- Introduction
- SARS-CoV-2
- Vaccine Emergency Use Authorization
- First COVID-19 Vaccine Authorized for Emergency Use
- Second COVID-19 Vaccine Authorized for Emergency Use
- Third COVID-19 Vaccine Authorized for Emergency Use
- COVID-19 Vaccine Ordering and Storing
- Pfizer-BioNTech COVID-19 Vaccine
- Moderna COVID-19 Vaccine
- Janssen COVID-19 Vaccine
- COVID-19 Vaccine Preparation and Administration
- Pfizer-BioNTech COVID-19 Vaccine
- Moderna COVID-19 Vaccine
- Janssen COVID-19 Vaccine
- Monitoring and Pre-Vaccination Screening
- Vaccine Adverse Event Reporting System
- Vaccine Side Effects
- Pfizer-BioNTech COVID-19 Vaccine
- Moderna COVID-19 Vaccine
- Janssen COVID-19 Vaccine
- Summary
Author
Kellie Wilson, PharmD
Kellie Wilson is a Doctor of Pharmacy practicing in Anaconda, Montana, where she lives with her husband and four children. She attended the University of Montana in Missoula where she graduated in 2009 with a doctorate in pharmacy. She later worked in Boise, Idaho for a large, retail pharmacy for 2 years, and then returned home to Montana to oversee an independently owned retail and long-term care pharmacy in Anaconda. As an independent retail pharmacist she has become very involved in psychiatric pharmacy for two major behavioral health organizations that are located around all of Montana. Kellie’s passion is retail pharmacy because she enjoys the interactions with customers as well as the challenges and rewards of staying current with the continuous changes in the pharmacy field.